
Solar-Recur Clinical Trial
A Phase 3, multicenter, open-label study to test the diagnostic performance of copper Cu 64 PSMA I&T PET/CT in men with biochemical recurrence of prostate cancer.
Solar-Recur
Study Overview
Solar-Recur is a single-arm prospective study. Local read of the copper Cu 64 PSMA I&T PET/CT can be used to inform patient management. For the primary endpoints, PET/CT scans will be read by blinded independent reviewers.
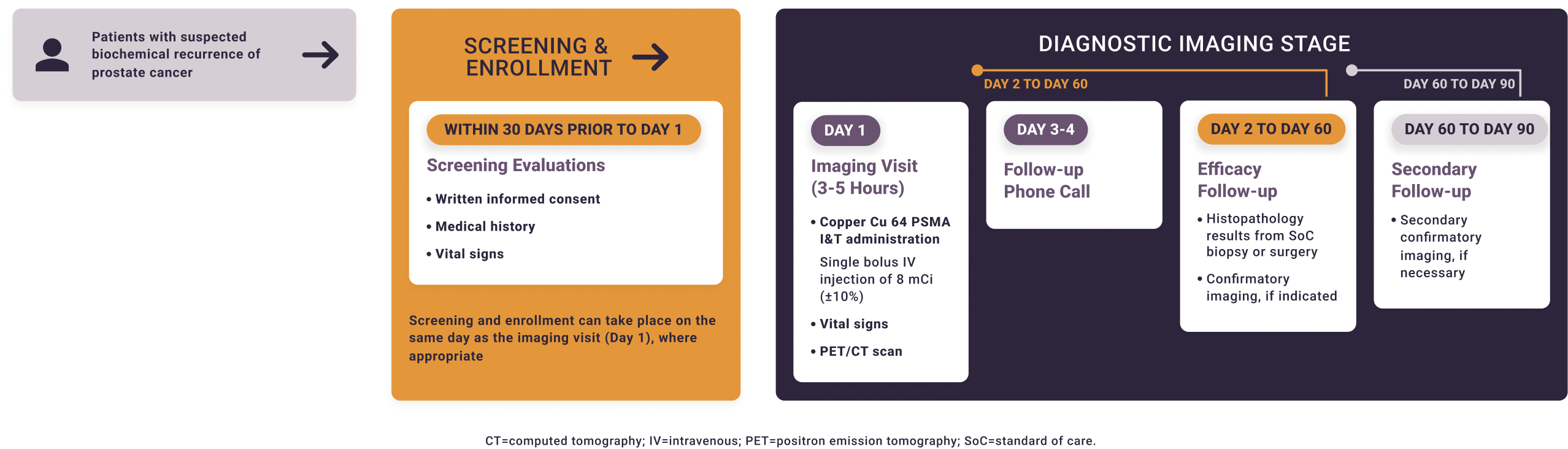
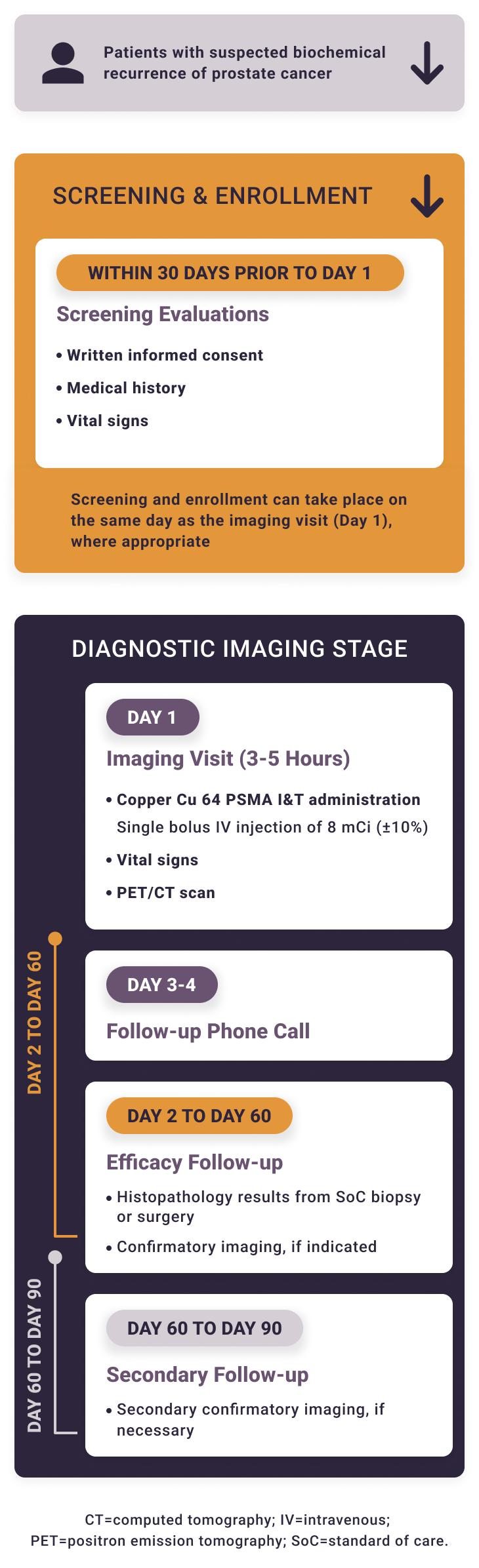
The Phase 3 studies of copper Cu 64 PSMA I&T, a radiopharmaceutical, are being performed for research purposes only. The safety and effectiveness of copper Cu 64 PSMA I&T have not been established by the United States Food and Drug Administration. The medical benefits and risks of this agent are under investigation.
Key Endpoints
- The Correct Detection Rate (CDR) of copper Cu 64 PSMA I&T PET/CT; i.e., the percentage of patients with at least 1 true positive lesion
- The Correct Localization Rate (CLR) of copper Cu 64 PSMA I&T PET/CT; i.e., the percentage of body regions containing at least 1 true positive lesion


- Patient-level CDR and region-level CLR of copper Cu 64 PSMA I&T PET/CT stratified by prostate-specific antigen (PSA) level
- Patient-level CDR and region-level CLR of copper Cu 64 PSMA I&T PET/CT separated into subgroups of patients with histopathology available and unavailable
- Inter- and intra-reader agreement of copper Cu 64 PSMA I&T PET/CT scan interpretation by blinded independent readers
- Treatment-emergent adverse events within 72 hours of copper Cu 64 PSMA I&T PET/CT administration
Key Eligibility Criteria
Key Inclusion Criteria
- Male aged ≥18 years
- Histologically proven prostate adenocarcinoma
- Prior RP (>6 weeks prior to screening) or radiation therapy (>1 year prior to screening) with curative intent
- Biochemical recurrence of prostate cancer
- For patients with prior RP: PSA >0.2 ng/mL followed by subsequent confirmatory PSA value >0.2 ng/mL, or
- For patients with prior radiation therapy: 2 ng/mL rise in PSA over post-treatment nadir
Key Exclusion Criteria
- Androgen deprivation therapy (ADT) or other therapies targeting the androgen pathway within the past 3 months. Patients with a rising PSA level while on ADT for >6 months are eligible
- PSMA PET within 90 days prior to enrollment
Study Sites
The Solar-Recur study will be conducted at sites across the United States. Click on each state below to see the trial locations and primary investigators.
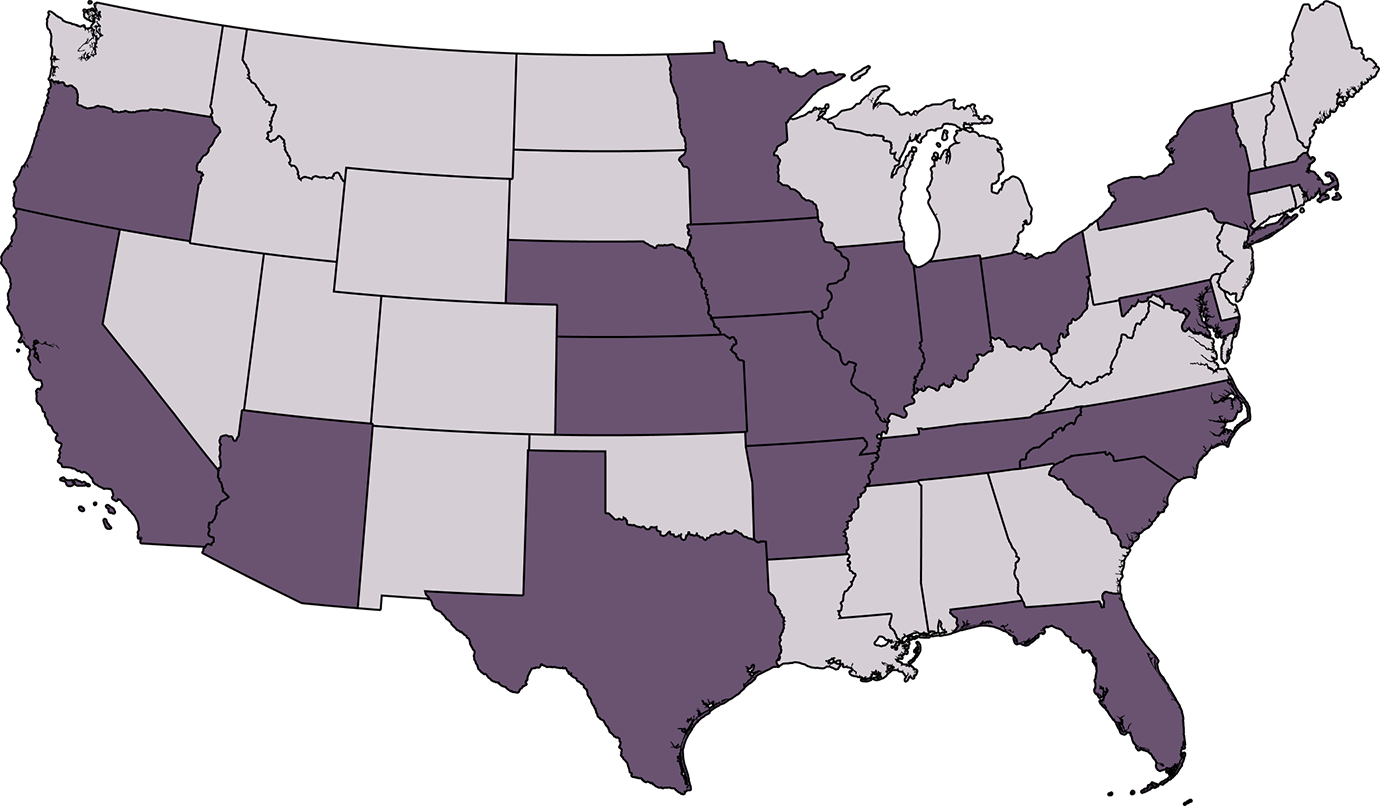
Click on each state below to see the trial locations and primary investigators.
Contact Us
For more information on eligibility and enrollment in the Solar-Recur clinical trial, please contact us using the form below.
More information is also
available at ClinicalTrials.gov
(Solar-Stage: NCT06235151,
Solar-Recur: NCT06235099).